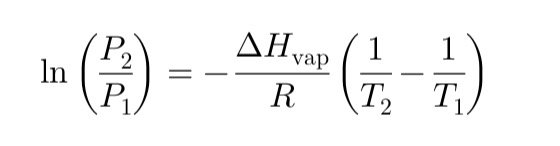Having the formula available on the blockchain is a great asset to society. !BBH
You are viewing a single comment's thread from:
Having the formula available on the blockchain is a great asset to society. !BBH
Thank you for your kind words of encouragement. In this post dedicated to chemistry I talked about vapor pressure. Vapor pressure is the pressure exerted by the vapor of a substance in equilibrium with its liquid at a given temperature. By increasing the temperature, the vapor pressure also increases, since more molecules are able to pass into the gaseous state.
One of the most well-known formulas that describes this conversion is the Clausius-Clapeyron equation that I reproduce in this image: